what does the electron cloud model describe|Electron Cloud Model : Pilipinas The electron cloud model is a theory that describes electrons as being part of a cloud around the nucleus of an atom, instead of being point-like particles. It . *Lotto and EuroMillions jackpots are estimated. Σ Each European Millionaire Maker Prize is €1,000,000 which, for UK winners, will be converted to Sterling and topped up by Allwyn from the UK Millionaire Maker Reserve Fund so that the total prize awarded is £1,000,000.
PH0 · What is the Electron Cloud Model: this is how
PH1 · What Is an Electron Cloud?
PH2 · What Is The Electron Cloud Model?
PH3 · Electron cloud
PH4 · Electron Cloud: Definition, Model, Explanation And Examples
PH5 · Electron Cloud: Definition, Model, Explanation And Examples
PH6 · Electron Cloud: Definition and Diagram
PH7 · Electron Cloud Model
PH8 · Electron Cloud
PH9 · Atomic orbital
As we look at what’s in store for the Rockstar Games community in the weeks and months ahead, we’d like to take this opportunity to thank everyone for their unmatched enthusiasm and commitment to all our titles. It’s your support that drives us to keep.
what does the electron cloud model describe*******The electron cloud is a cloud of probability surrounding the nucleus in an atom where one has the highest probability of finding an electron. When you think of an atom, your mind probably conjures up an image of a central nucleus with a whole . Learn about electron cloud model, where is the electron cloud located, who discovered the electron cloud and its theory along with diagram.The electron cloud model is a theory that describes electrons as being part of a cloud around the nucleus of an atom, instead of being point-like particles. It .
Learn what an electron cloud is, how it describes the behavior of electrons in atoms, and what theories explain its quantum nature. See examples, diagrams, . Anne Marie Helmenstine, Ph.D. Updated on December 08, 2019. An electron cloud is the region of negative charge surrounding .
What is the Electron Cloud Model: this is how electrons inside an atom really behave. From the ancient Greeks to quantum mechanics, the model of the atom has gone through many.
The electron cloud is also defined as the region where an electron forms a three-dimensional standing wave, the one that does not move relative to the atomic nucleus. .
Atomic orbitals are the basic building blocks of the atomic orbital model (or electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this .
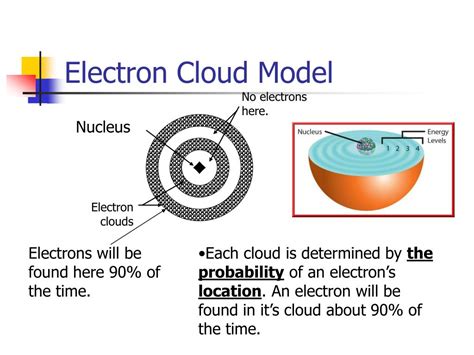
The electron cloud model says that we cannot know exactly where an electron is at any given time, but the electrons are more likely to be in specific areas. These areas are .The electron cloud model describes the atom as a nucleus surrounded by a cloud of rapidly moving electrons with a zone of probability. Learn how the model explains the uncertainty principle, the . A crash course in electron behavior.—More on the Atomic Model | Wiki—"In quantum mechanics, an atomic orbital is a mathematical function that describes the w.
what does the electron cloud model describeErwin Schrödinger proposed the quantum mechanical model of the atom, which treats electrons as matter waves. , represents the probability of finding an electron in a given region within the atom. An atomic orbital is defined as the region within an atom that encloses where the electron is likely to be 90% of the time. The model is a tool for visualising the most likely electron positions in an atom. The electron cloud model is now the most widely known atom model. Since the electrons were moving so quickly, there was no way of knowing where or when the electron would appear. The electrons were like a blur, which is how they came up with .The electron cloud model defines the zone of probability describing the electron’s location because of the uncertainty principle. The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means .Electron Cloud Model. The Electron Cloud Model. It tells us that electrons are found in defined energy states in outside of the nucleus of the atom. The lowest possible state for any electron is called its ground state. These energy levels can only hold a certain number of electrons at any time and the organizations of these electrons in the . What does the electron cloud model describe? A. the most likely locations of electrons in atoms B. the precise locations of electrons in atoms C. the number of electrons in an atom D. the mass of the electrons in an atomPreview. 26 terms. Samrmartin14. Preview. Study with Quizlet and memorize flashcards containing terms like What does the electron cloud model describe?, Greek philosopher Democritus coined what word for a tiny piece of matter that cannot be devided?, An electron has a far less mass than either a proton or neutron. True or False? and more.
The electron cloud atomic model and atomic orbitals. Click Create Assignment to assign this modality to your LMS. We have a new and improved read on this topic. Click here to view We have moved all content for this concept to for better organization. Please update your bookmarks accordingly.
An atomic orbital, which is distinct from an orbit, is a general region in an atom within which an electron is most probable to reside. The quantum mechanical model specifies the probability of finding an electron in the three-dimensional space around the nucleus and is based on solutions of the Schrödinger equation.The electron cloud model describes the distribution of electrons in an atom. According to this model, electrons are not in fixed orbits, as previously thought in the planetary model. Instead, the electron cloud represents the probabilistic locations that an electron may be found around the nucleus. The correct answer to the question is C.
The electron cloud model says that we cannot know exactly where an electron is at any given time, but the electrons are more likely to be in specific areas. Electron cloud model defines the zone of probability describing the electron’s location, because of the uncertainty principle. The electrons in an atom are attracted to the . Electrons do not travel around the nucleus in simple circular orbits. The location of the electrons in the quantum mechanical model of the atom is often referred to as an electron cloud. The electron cloud can be thought of in the following way: Imagine placing a square piece of paper on the floor with a dot in the circle representing the .A strength of this model is how it represents the wave behavior of electrons. The fuzzy electron cloud represents how individual electrons are actually spread out through space. Until we measure an electron's position, we don't know exactly where it is. The best we can do is describe where we're likely to find electrons around a nucleus .
The location of the electrons in the quantum mechanical model of the atom is often referred to as an electron cloud. The electron cloud can be thought of in the following way: Imagine placing a square piece of paper on the floor with a dot in the circle representing the nucleus. Now take a marker and drop it onto the paper repeatedly, .
The electron cloud model is the current model we use to describe atoms. It refers to the cloud of electrons surrounding the nucleus of the atom. 1 Answer. Electron cloud model describes the most probable location of an electron is. It does not say the exact location of the electron as it is moving continuously. Electron cloud model describes the most probable location of an electron is.

Figure 22.15 The ground state of a hydrogen atom has a probability cloud describing the position of its electron. The probability of finding the electron is proportional to the darkness of the cloud. The electron can be closer or farther than the Bohr radius, but it is very unlikely to be a great distance from the nucleus.
what does the electron cloud model describe Electron Cloud Model Figure 22.15 The ground state of a hydrogen atom has a probability cloud describing the position of its electron. The probability of finding the electron is proportional to the darkness of the cloud. The electron can be closer or farther than the Bohr radius, but it is very unlikely to be a great distance from the nucleus.Electron Cloud Model By CK-12. Common Core Math. College FlexBooks. K-12 FlexBooks. Tools and Apps.
Scribd is the world's largest social reading and publishing site.
what does the electron cloud model describe|Electron Cloud Model